Patients participated in testing of PRIDD Measure
The Global Research on the Impact of Dermatological Diseases (GRIDD) project is nearly is now in the final stages of Phase 4 where we tested the pilot version of the Patient-Reported Impact of Dermatological Diseases (PRIDD) measure.
In July and August 2022, GlobalSkin invited our Members and their patient communities from a broad cross-section of dermatological diseases and regions, to be involved in the final psychometric testing of the PRIDD measure.
DERMATOLOGY PATIENTS ARE ESSENTIAL AS THEY ARE DISEASE EXPERTS!
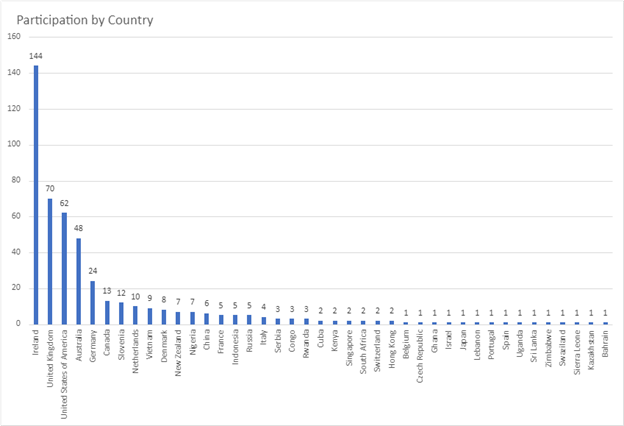
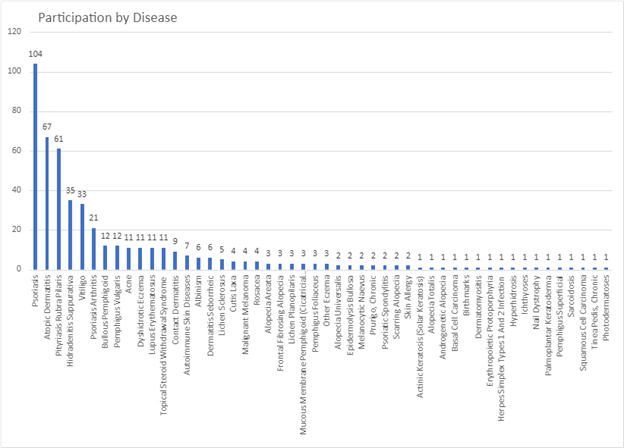
Over 500 dermatology patients from 33 dermatological diseases in 38 countries took part in two surveys approximately two weeks apart. The surveys available in english only, included PRIDD and a few other validated questionnaires as well as some questions about the patients, such as age, gender, which dermatological condition and where the patient lived. This study tested the reliability of the pilot PRIDD measure over time.
Participation by country
Participation by disease
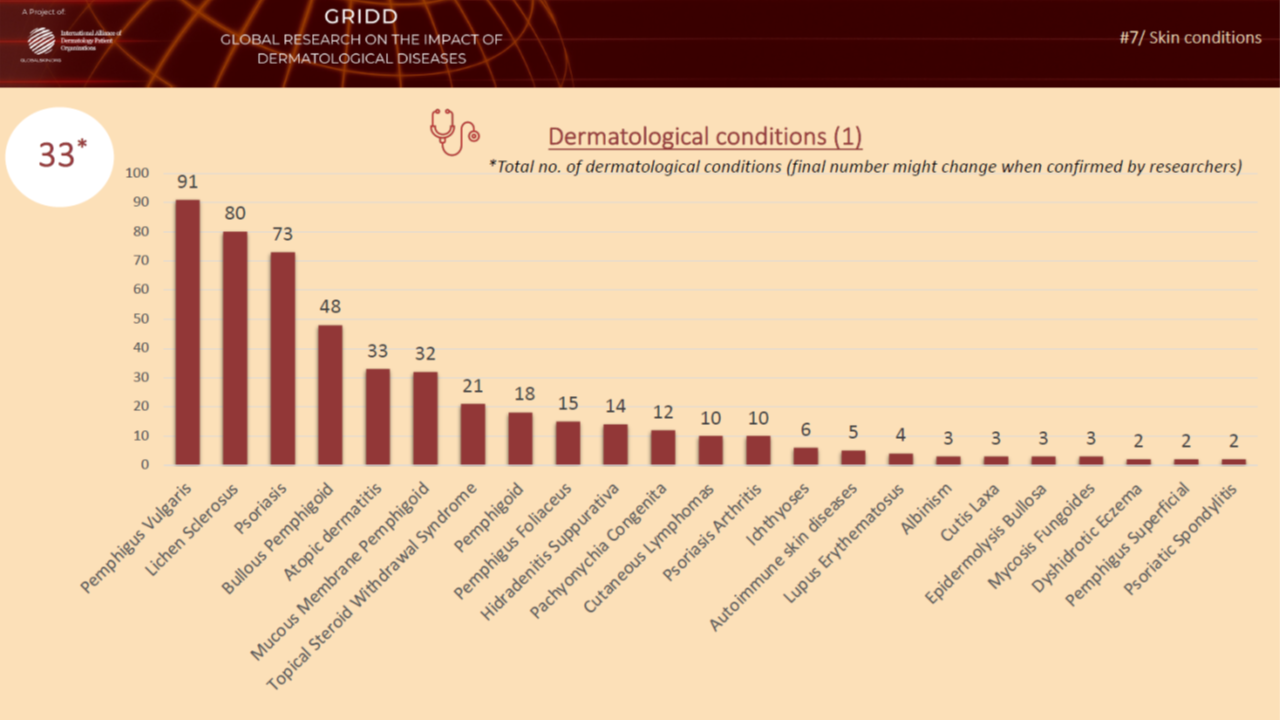
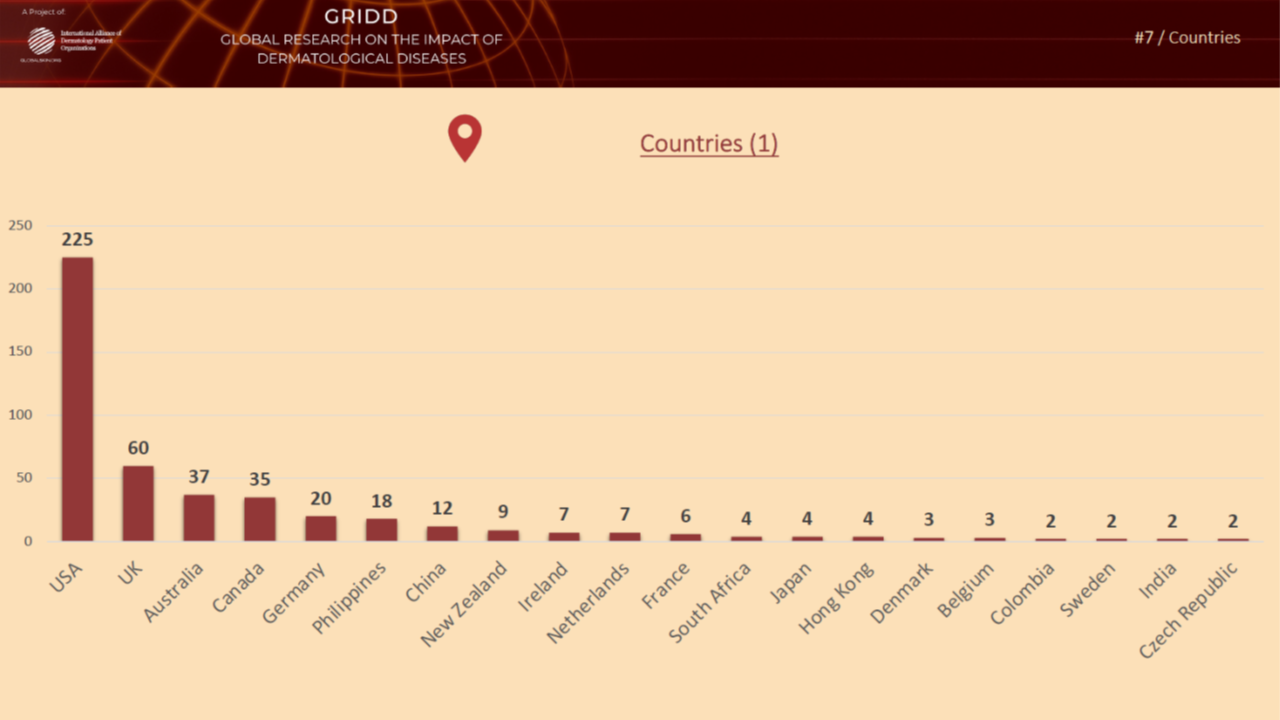
Previous psychometric testing during Phase 4
In November 2021, we tested the pilot version of the Patient-Reported Impact of Dermatological Diseases (PRIDD) measure. We were pleased to have heard from 486 patients located in 31 countries, representing 49 dermatological diseases. We met our targets with this diverse patient participation. The Research team will now analyze the data and findings from this psychometric testing and make any refinements to PRIDD as needed. The images below illustrate patient participation by country and disesase area during the Novmeber testing.
A big thank you to our Members for your support in getting your patients involved with the GRIDD project – without your endorsement, we could not have come so far in this ground-breaking patient-led research project.
Phase 3 Delphi - Explained
In late 2020 and 2021, the Global Research on the Impact of Dermatological Diseases (GRIDD) team actively engaged patients around the world in a Delphi process, a key step in developing the Patient-Reported Impact of Dermatological Diseases (PRIDD) measure. Thank you to our patient organizations around the world for their help in making the GRIDD Delphi process a success!
A Delphi is a consensus-seeking method widely used in health research. The main purpose of a Delphi is to explore a topic in a way that goes beyond what is currently known or believed and is based on the assumption that expert group judgments are more valid or accurate than individual judgments or assumptions. It is a scientific process that promotes greater accuracy and validates previous data-derived assumptions.
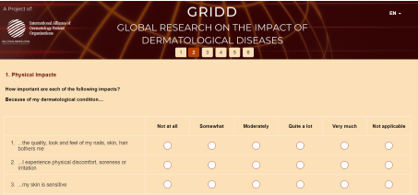 The GRIDD Delphi was comprised of two consecutive surveys, targeted at adults (over 18 yrs.) diagnosed with a dermatological condition. The surveys were available in multiple languages: English, French, Spanish, German, Mandarin (simplified Chinese), and Arabic.
The GRIDD Delphi was comprised of two consecutive surveys, targeted at adults (over 18 yrs.) diagnosed with a dermatological condition. The surveys were available in multiple languages: English, French, Spanish, German, Mandarin (simplified Chinese), and Arabic.
On June 3, the GRIDD Delphi concluded. In Delphi #1, which took place December-January, 1154 patients from 61 countries, representing 90 diseases completed 263 questions about the impact of their dermatological diseases. In Delphi #2, which took place May-June, 493 of the original respondents completed the significantly refined and reduced 76-question questionnaire.
Results of the Delphi, along with previous GRIDD research, identified multiple important ways that dermatological conditions affect patients’ lives. We want to understand how important each of these impacts are to patients’ quality of life.
This information is essential information for the development of PRIDD. This new measurement tool will allow us to collect data that shows what it is really like to live with a dermatological condition, thus helping dermatology patient organizations advocate for better access to treatments and appropriate healthcare for patients globally.
Item reduction is now complete (~33 items down from 76 items in Delphi 2 and 263 items in Delphi 1) and the pilot version of PRIDD is in currently undergoing review and approvals.