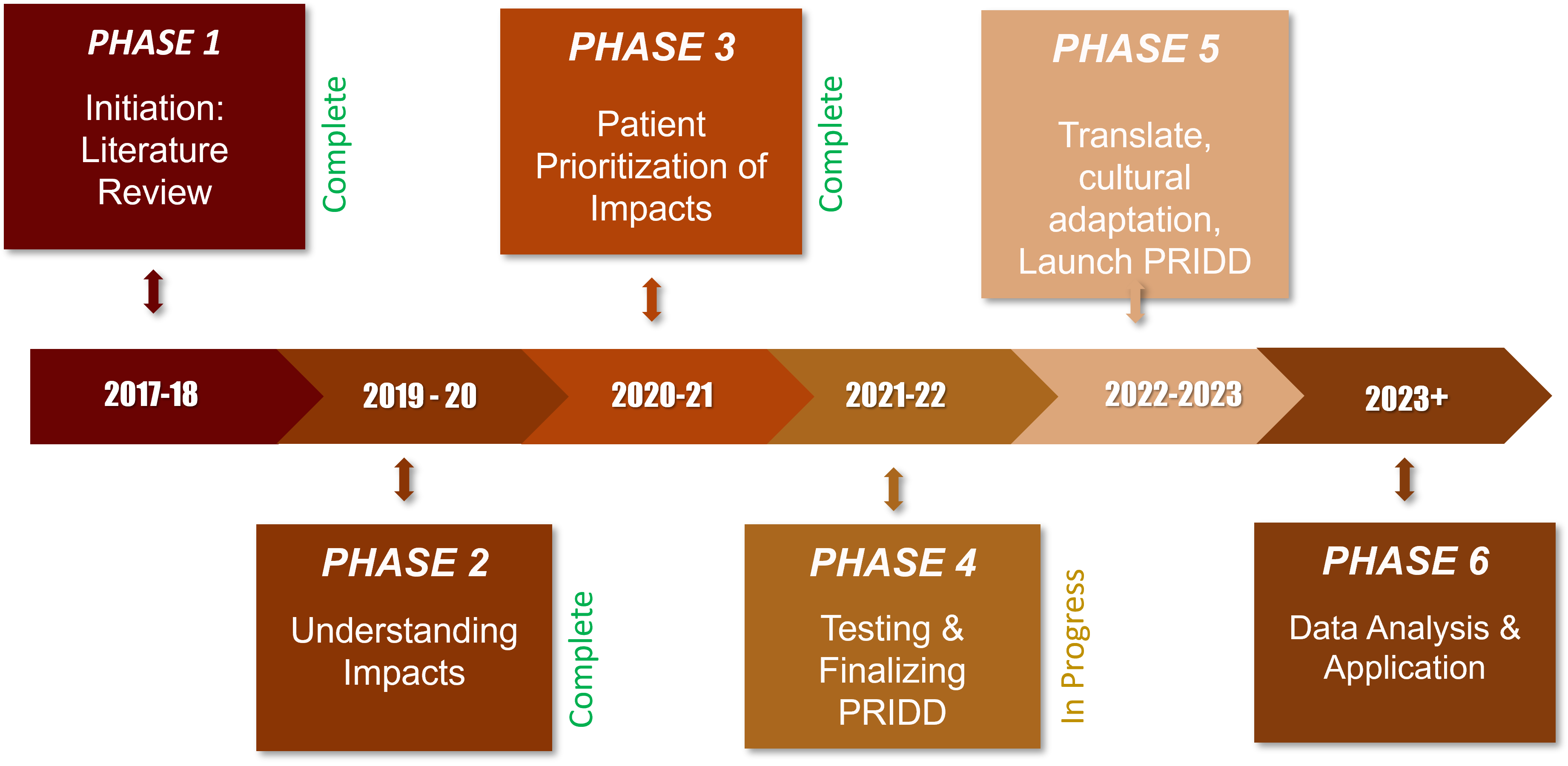
The GRIDD project, a multi-year, multi-phases project will produce benefits that will endure for generations to come.
The implementation of each phase will result in a new GRIDD methodology called Global Research of Impact on Patients (GRIP), a novel Patient-Reported Impact of Dermatological Diseases (PRIDD) measurement tool, as well as a data that will help transform the way the world sees and addresses dermatological diseases.
Learn about all the phases below and see where we are today!
Click to expand the below table by each phase.
GRIDD Project Timeline: 2017 – 2024
Phase 1
|
2017-2018
|
MAIN OUTCOMES |
|
Systematic Literature Review - Completed |
|
|
|
|
|
|
|
|
|
|
|
|
The results from GRIDD Phase 1 Systematic Literature Review (2017-2018) found that existing patient-impact measurement tools (questionnaires) used in both dermatology research and clinical settings don’t meet the commonly accepted scientific standards. This means there is currently no COSMIN A Standard for dermatology.GRIDD will address this gap with PRIDD: the A Standard patient-impact measurement tool that will provide the data for many applications including elevating dermatology in the global disease rankings; |