The Global Research on the Impact of Dermatological Diseases (GRIDD) team has actively engaged patients around the world during the Phase 3 Delphi process. This phase was a key step in developing the Patient-Reported Impact of Dermatological Diseases (PRIDD) measure. Thank you to our patient organizations around the globe for their help in making the GRIDD Delphi process a success!
This new measurement tool will allow us to collect data that shows what it is really like to live with a dermatological condition, thus helping dermatology patient organizations advocate for better access to treatments and appropriate healthcare for patients globally.
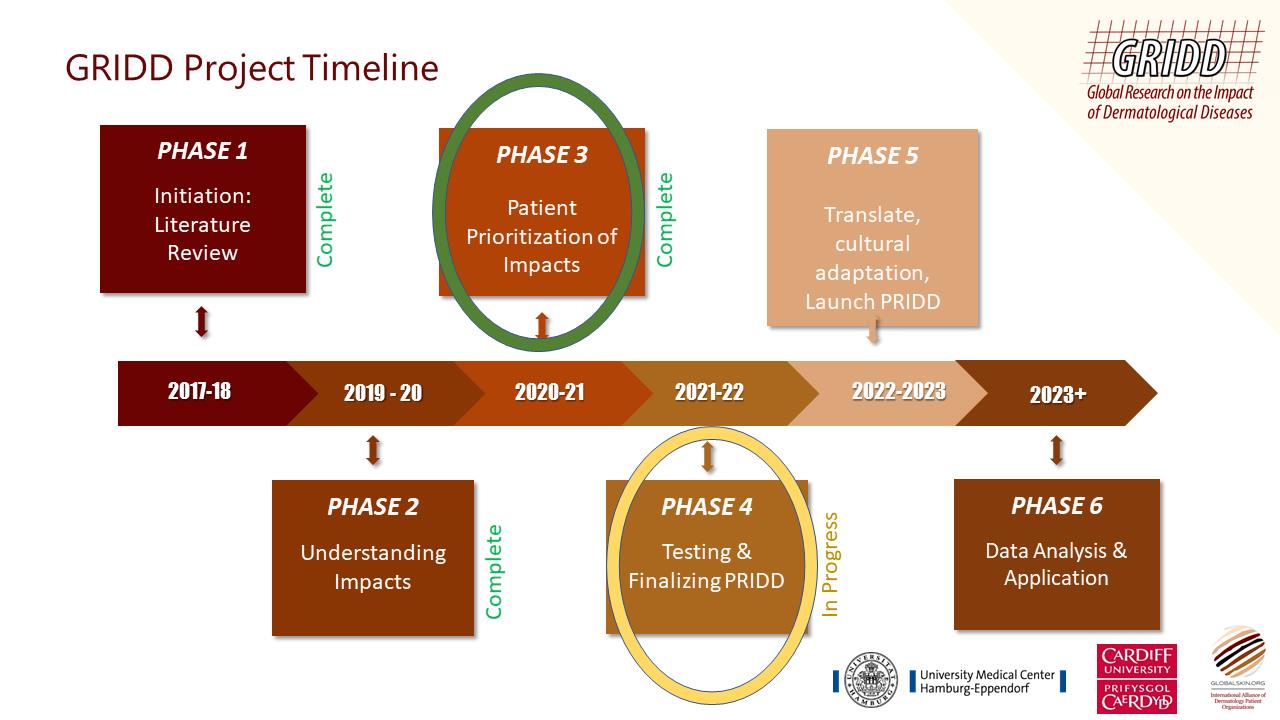
We now have a pilot PRIDD tool and have entered Phase 4 - Testing and Finalizing PRIDD.
Next Steps:
1. Cognitive Interviews
Cognitive interviews took place in Julys-August 2021 where 15 patients tested the comprehensibility, relevance, comprehensiveness, acceptability, and feasibility of PRIDD. The data gathered from patients was used to test how PRIDD performs as a statistical tool and make refinements accordingly.
2. Psychometric Testing:
Statistical refinements: The data gathered from patients will be used to test how PRIDD performs as a statistical tool and make refinements accordingly.
What this means (November 2021): GlobalSkin is looking for (up to) 500 patients to participate in Psychometric Testing. Patient organizations will be asked to encourage their patients to participate in the online survey.
Testing of measurement properties: PRIDD’s quality will be established through its measurement properties to understand what scores mean.
What this means: (Early-2022): All patient organizations will be asked to encourage their patients to participate in Psychometric Testing surveys – test/re-test.
3. Translation
Translation will take place after the completion and validation of the PRIDD measure (back translation and validation).



